
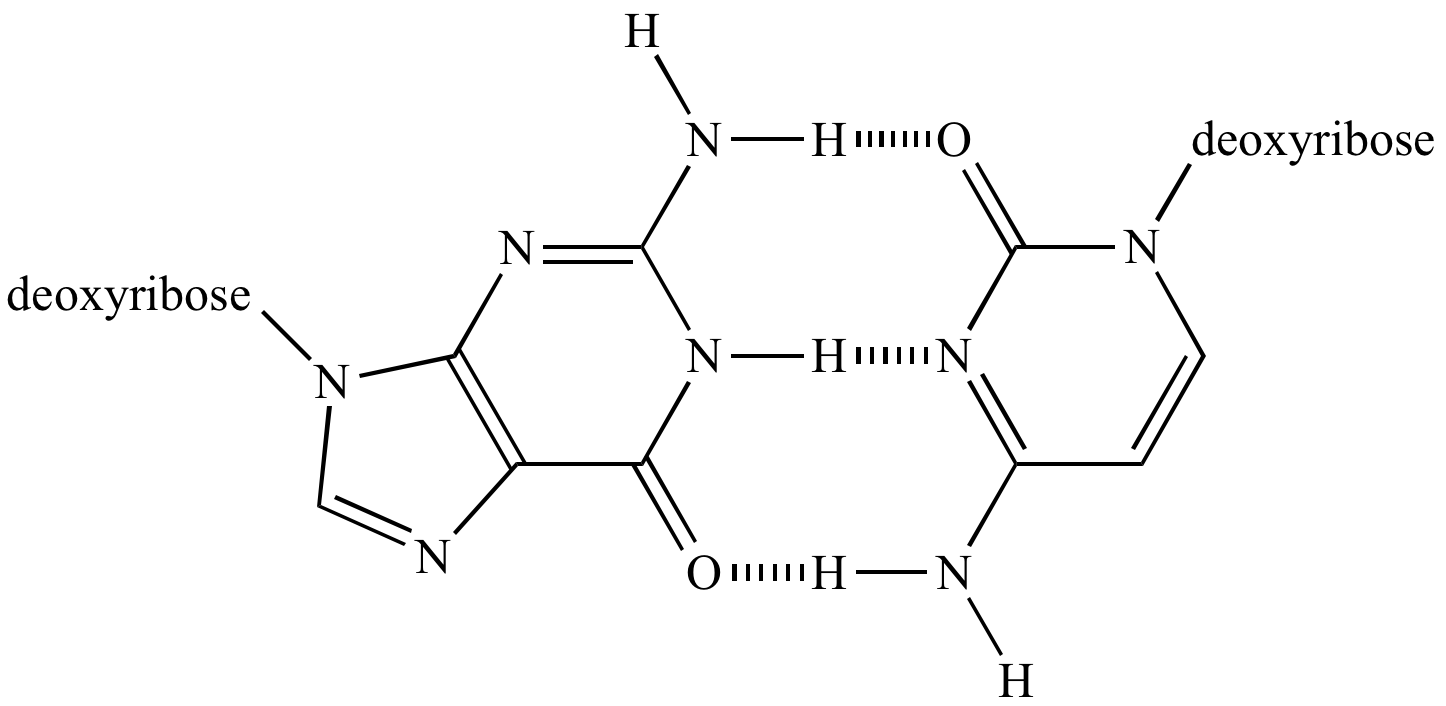
The hydrogen bonds are formed between the oxygen atoms (red) and the adjacent N-H bonds, and between the central nitrogen (blue) and the adjacent N-H bond. According to the Watson-Crick model, the double helix of DNA is assembled and stabilized by hydrogen pairing between matching “bases”. Hydrogen bonding is also important is DNA. The extra energy that is required is necessary to break down the hydrogen bonding network. Although these two compounds have similar molar masses, a significant amount of energy must be put into the polar molecule, water, in order to move into the gas phase, relative to the non-polar methane. Water, with a molar mass of 18, has a boiling point of +100 ˚C. Methane, CH 4, has a molar mass of 16 and a boiling point of –164 ˚C. For many compounds which do not possess highly polarized bonds, boiling points parallel the molar mass of the compound. Hydrogen bonding is generally used to explain the high boiling point of water (100 ˚C). Even though hydrogen bonds are relatively weak, if you consider that every water molecule is participating in a least four hydrogen bonds, the total energy of hydrogen bonding interactions can rapidly become significant.
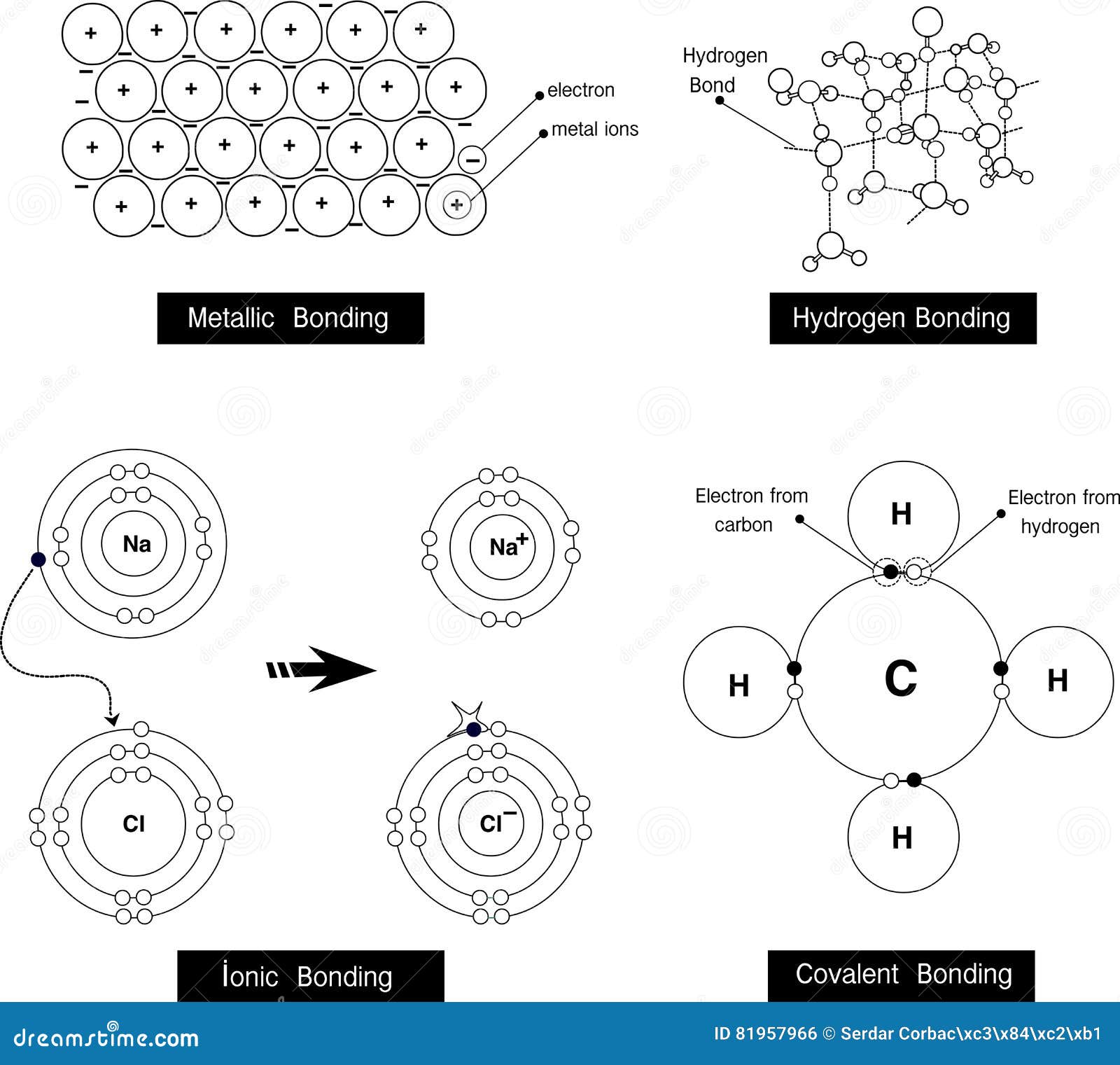
Hydrogen bonds are weak, relative to covalent bonds. Recall that oxygen has two unshared pairs in its valence shell, and the hydrogen-oxygen interaction in water is the classic example of a hydrogen bond. A bond that is formed from a hydrogen atom, which is part of a polar covalent bond (such as the O-H bond) to another, more electronegative atom (that has at least one unshared pair of electrons in its valence shell) is called a hydrogen bond. By this logic, it is not at all surprising that water can also react strongly with itself, and indeed water exists as a vast network of molecules aligned so that their positive and negative dipoles interact with each other. We attributed this to the ability of water molecules to align themselves so that the polarized hydrogen-oxygen bonds could stabilize cations, anions, and virtually any compound that also contained a significantly polarized covalent bond. In Chapter 7, we explored the unique properties of water that allow it to serve as a powerful solvent with the ability to dissolve both ionic compounds, as well as polar molecular compounds. However, the ν(X-H) is shifted to lower energy (lower frequency) as a consequence of hydrogen bonding.\)

The X-H stretching frequency in the IR (and Raman) spectrum is dependant on the identity of X, i.e., O-H = 3610 - 3640 cm -1 and N-H = 3400 - 3500 cm -1. In contrast, the value typically seen for a hydrogen-bonded analog is 1.05 Å. Also, you will learn that hydrogen bonding exists in ammonia, water and Hydrogen fluoride.Q. For example the O-H distance for an alcohol in the absence of hydrogen bonding is typically 0.97 Å. In his animated lecture, I will teach you about hydrogen bonding. The bond distance to hydrogen, d(X-H), is often longer in hydrogen bonded species. \).5: Comparison of the X.Y distance in hydrogen bonded species with the sum of the van der Waal radii.


 0 kommentar(er)
0 kommentar(er)
